Pipeline
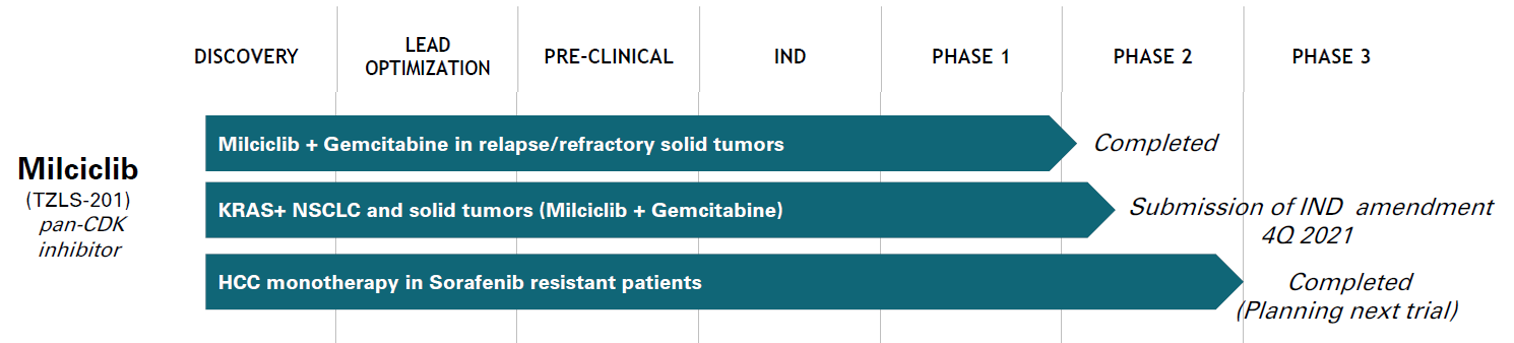
- To date, Milciclib has been studied in a total of eight completed and ongoing Phase 1 and 2 clinical trials in 316 patients.
- In these trials, Milciclib was observed to be well-tolerated and showed initial signals of anti-tumor action.
- Milciclib was granted orphan designation by the European Commission and by the U.S. Food and Drug Administration (FDA) for the treatment of thymic carcinoma.
In two Phase 2a trials, CDKO-125a-006 and CDKO125a-007 (https://clinicaltrials.gov/ct2/show/NCT01301391 and https://clinicaltrials.gov/ct2/show/NCT03109886), Milciclib exceeded efficacy endpoints, showed signs of slowing disease progression and acceptable safety.