Learn more about Tiziana Tiziana Investor Deck Scientific Presentations and Updates
23 April 2024
NEW YORK, April 23, 2024 – Tiziana Life Sciences, Ltd. (Nasdaq: TLSA) (“Tiziana” or the “Company”), a biotechnology company developing breakthrough immunomodulation therapies via novel routes of drug delivery, today announced that the U.S. Food and Drug Administration (FDA) has...
Read more22 April 2024
19 April 2024
Our mission is to bring breakthrough therapies to patients with the aim of treating Secondary Progressive Multiple Sclerosis, ALS, Alzheimer's, and other CNS indications. Crohn's Disease, lung diseases and optimizing health outcomes.
Learn moreOur major clinical assets are supported by extensive worldwide issued patents and pending patent applications covering composition of matter, formulation technologies, manufacturing processes and disease indications.
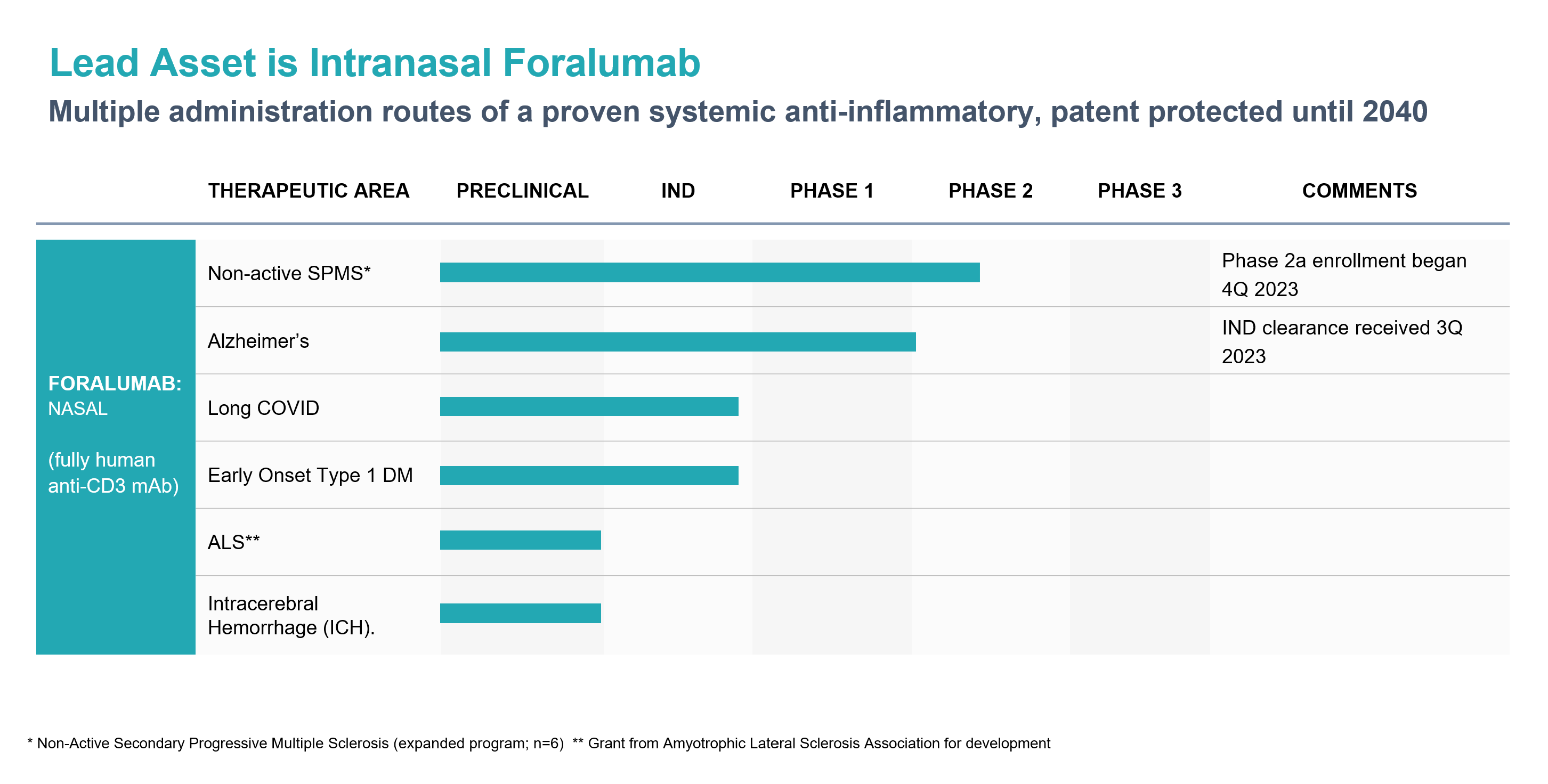
Foralumab is a fully human anti-CD3 monoclonal antibody (mAb) for treatment of Crohn’ s Disease and neurodegenerative indications.
Learn more about ForalumabWe are developing a fully human monoclonal antibody (mAb) targeting the receptor for IL-6 as a potential treatment for lung diseases.
Learn more about Anti IL-6RMilciclib is the Company’s clinical candidate for the treatment in cancer indications. The company is exploring the combination of milciclib and gemcitibine in NSCLC patients with pan KRAS+ mutations.
Learn more about MilciclibTiziana is currently conducting clinical development programs for Foralumab, Anti IL-6R and Milciclib
Tiziana reported positive clinical data in a Phase I clinical trial of nasally-dosed Foralumab in healthy subjects in collaboration with Dr. Howard Weiner at Brigham and Women’s Hospital in Boston. Expanded Access program now dosing 6 patients. Phase 2 patient enrollment is expected to begin in 2H 2023 for nasally administered Foralumab for the treatment of Non-active, Secondary Progressive, Multiple Sclerosis.
See more Foralumab clinical trialsManufacturing of clinical supplies for a Phase 1 study is anticipated to be completed in 4Q 2022.
We are exploring a study to evaluate the combination of milciclib and gemcitabine in NSCLC subjects with associated pan KRAS-positive mutations.
See more Milciclib clinical trials